- Share
- Share on Facebook
- Share on X
- Share on LinkedIn
Objectives
Many food products are present in the form of colloidal suspensions dispersed in an aqueous phase. The interactions between these colloids play a key role in controlling the rheological and nutritional properties of foods. Among them, proteins occupy a particular place, as their conformation and reactivity are strongly influenced by physicochemical, thermal, and mechanical conditions. This sensitivity complicates separation processes but also opens new perspectives for assembling food proteins into innovative functional ingredients.
Our experimental approach, strongly based on the development of elementary processes coupled with advanced imaging techniques at different scales (microscopy, radiation scattering), has enabled us to identify the rheophysical mechanisms (i) limiting the separation processes of casein micelle suspensions and (ii) governing the assembly of dairy and plant proteins during structuring processes.
Results
Mechanisms of accumulation and relaxation of casein micelles during milk tangential filtration
Microfiltration and ultrafiltration are separation processes widely used in the dairy industry, but they remain poorly controlled, in particular due to the formation of a concentrated and gelled layer of casein micelles near the membrane. Our objective was to understand the physical, rheological, and cohesive properties of casein micelles accumulated at the membrane surface during tangential filtration under different operating conditions, and more specifically under the effect of temperature (PhD thesis of Floriane Doudiès, collaboration with STLO / INRAE).
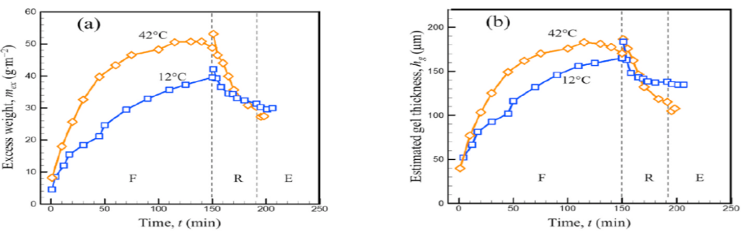
The study of rheological behavior as a function of temperature showed that casein micelle gels are less compressible at low temperatures (7 and 12 °C) than at higher temperatures (20, 25, and 42 °C), leading to a lower sol–gel transition concentration and stronger cohesion between casein micelles. As a result, when temperature decreases, the gelled deposits at the membrane surface are thicker and reduce filtration performance (Doudiès et al., 2019).
Through an in-situ SAXS approach performed during filtration, it was shown that it is possible to partially remove deposits formed during tangential and frontal filtrations by releasing the applied pressure. It was demonstrated that during the relaxation phase and erosion induced by tangential flow, the swollen deposit obtained at 42 °C was eroded more extensively than that obtained at 12 °C (Doudiès et al., 2021; Loginov et al., 2020, 2021). This highlights higher filtration performance at elevated temperatures, while also leading to the formation of deposits with lower cohesion, which would then be more easily removed during cleaning operations.
Aggregation and gelation of whey proteins under flow
A co-product of milk filtration processes, whey protein isolate (WPI) is a mixture of mineral salts and two native proteins, β-lactoglobulin and α-lactalbumin. These two proteins have the particular property of denaturing at high temperatures (>80 °C), leading to the formation of fractal aggregates under neutral pH conditions. This fractal structure is of particular interest for applications in food texturing. Since the technological routes for creating these particles with thickening properties are mainly based on modulating physicochemical conditions during aggregation, the objective of our research (PhD thesis of Alice Vilotte) was to identify new physical pathways of aggregation and gelation of WPIs, with the aim of producing novel aggregate or gel structures.
We identified the parameters controlling the aggregation kinetics of WPI aggregates in thermally activated aggregation processes (Vilotte et al., 2020). To achieve this, we developed a downscaling approach to aggregation using a microcapillary heated at high temperature (92 °C). This microfluidic approach eliminates limitations due to heat transfer. We coupled this approach with structural characterization by small-angle X-ray scattering (ESRF, Grenoble). The scattering spectra allowed us to simultaneously monitor aggregate growth at submicrometric scales and denaturation kinetics at the protein scale. This provided access to the three key steps of the aggregation process: denaturation, nucleation, and aggregation. We demonstrated that the size and mass of aggregates reach a steady-state value within a few seconds, although the timescale associated with the aggregation kinetics is on the order of a few minutes. Moreover, we observed that the kinetics of native protein consumption mirrored that of aggregated proteins, indicating that aggregation occurs as soon as proteins are denatured. This suggests that aggregation is limited by a nucleation step and that there exists a mechanism restricting aggregate size, independently of shear induced by flow.
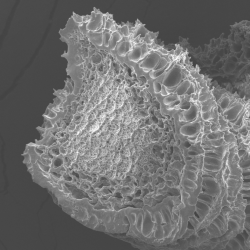
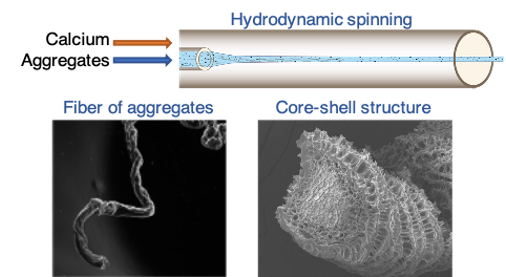
These WPI aggregates were subsequently assembled into fibers of approximately one hundred micrometers in diameter via a microfluidic process (Vilotte et al., 2022). This process consists in co-injecting a suspension of preformed WPI aggregates with a calcium chloride solution, which acts as a crosslinker between the aggregates. After identifying the relatively narrow hydrodynamic conditions that enable fiber formation, we highlighted different stability regimes, which vary non-monotonically with calcium chloride concentration. At low CaCl₂ concentration, fibers tend to swell with water until reaching a critical CaCl₂ concentration, beyond which they completely disperse, whereas their plant protein counterparts remain stable (Vilotte et al., 2023). This micro–phase separation mechanism is consistent with a syneresis phenomenon. More surprisingly, at high CaCl₂ concentration, we observed the formation of stable fibers exhibiting a protein concentration gradient: the fiber corona is more enriched in proteins than the core. This striking result motivated the postdoctoral work of Émilie Guilbert, aimed at identifying the physical phenomena governing this latter regime.
To this end, we investigated the ionic gelation mechanism of WPI aggregates by fluorescence microscopy in a simple experimental system (Guilbert et al., 2024).
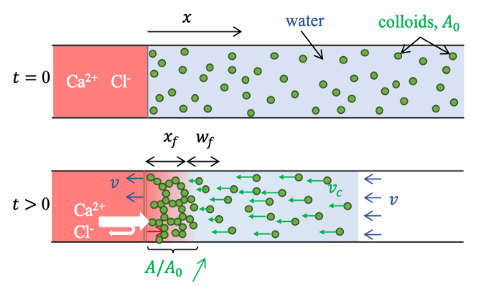
Bottom: Calcium chloride ions diffuse into the suspension (pink gradient) and induce its gelation over a distance xf, which increases over time. As the gel is partially selective to calcium chloride (white arrows), the osmotic pressure difference between the suspension and the CaCl₂ solution generates an osmotic flow v. This flow carries the ungelled colloids at a velocity v. Consequently, the colloid concentration increases in the transition zone between the gel and the liquid, leading to the formation of a colloid concentration gradient.
This setup consists of a glass capillary filled with the suspension to be gelled, one end of which is brought into contact with a drop of CaCl₂. Using this device, we observed that the solvent of the suspension percolates through the forming gel toward the CaCl₂ solution. This flow, proportional to the osmotic pressure of the calcium solution, suggests an osmotic origin. For such a flow to be established, the presence of a membrane partially selective to ions is necessary. By applying a negative pressure at the opposite end of the capillary, we were able to modulate this flow, up to its complete suppression.
Relying on the Kedem–Katchalsky relation, which links the difference in mechanical and osmotic pressure to the flux through a membrane as well as to its porosity and selectivity, we demonstrated that the forming gel behaves as a semipermeable membrane for ions.
We also generalized this mechanism to other hydrogels obtained by ionic gelation of polysaccharides, such as alginate and pectin. A surprising consequence of this effect, related to the formation of fibers with a concentration gradient, is the appearance of concentration gradients near the interface with the gelling solution.
Viscoelastic properties of bean seed proteins
We studied the effect of heat on the cooking of bean seeds in order to optimize their preparation conditions. The mechanical test simulates a finger‑pressing test on the seeds. In this study, the seed is considered as a system composed of water, protein, starch, and a residual amount of fat. This approach allows correlating protein denaturation mechanisms, starch gelatinization, and water and heat transfer with the measured viscoelastic and mechanical behavior (Teko et al., 2021).
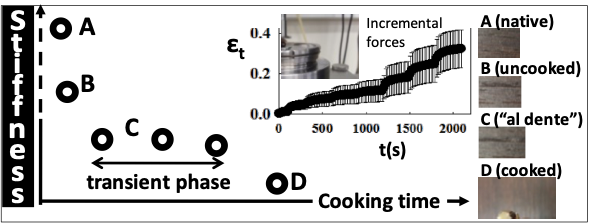
The study revealed a cooking time window at 90 °C during which the mechanical parameters (deformation) are nearly stable. The stability of these cooking parameters reflects an antagonistic balance between protein denaturation and starch gelatinization effects on mechanical behavior. We demonstrated how the thermal effect influences water transfer from the cooking medium to the seed. This water transfer affects both the cooking time and the viscoelastic behavior of the seed.
References
Doudiès, F., Arsène, A. S., Garnier-Lambrouin, F., Famelart, M. H., Bouchoux, A., Pignon, F., & Gésan-Guiziou, G. (2019). Major role of voluminosity in the compressibility and sol–gel transition of casein micelle dispersions concentrated at 7 C and 20 C. Foods, 8(12), 652.
Doudiès, F., Loginov, M., Hengl, N., Karrouch, M., Leconte, N., Garnier-Lambrouin, F., ... & Gésan-Guiziou, G. (2021). Build-up and relaxation of membrane fouling deposits produced during crossflow ultrafiltration of casein micelle dispersions at 12° C and 42° C probed by in situ SAXS. Journal of Membrane Science, 618, 118700.
Loginov, M., Doudies, F., Hengl, N., Pignon, F., & Gésan-Guiziou, G. (2020). Influence of membrane resistance on swelling and removal of colloidal filter cake after filtration pressure release. Journal of Membrane Science, 595, 117498.
Vilotte, A., Bodiguel, H., Ako, K., Gunes, D. Z., Schmitt, C., & de Loubens, C. (2021). Kinetic and structural characterization of whey protein aggregation in a millifluidic continuous process. Food Hydrocolloids, 110, 106137.
Vilotte, A., de Loubens, C., Gunes, D. Z., Schmitt, C., & Bodiguel, H. (2022). Hydrodynamic spinning of protein fractal aggregates into core–shell fibers. ACS Applied Polymer Materials, 4(6), 4075-4080.
Vilotte, A., Bodiguel, H., Gunes, D. Z., Schmitt, C., Roux, D., Guilbert, E., ... & de Loubens, C. (2023). Formation and stability of fibers obtained by cold gelation of pea protein isolate aggregates in a hydrodynamic spinning process. Food Hydrocolloids, 145, 108999.
Guilbert, E., de Loubens, C., Vilotte, A., Schmitt, C., Gunes, D., & Bodiguel, H. (2024). Spontaneous Structuration of Biohydrogels by Membrane‐Free Osmosis. Advanced Functional Materials, 34(34), 2400888.
Teko, E., Osseyi, E., Munialo, C. D., & Ako, K. (2021). The transitioning feature between uncooked and cooked cowpea seeds studied by the mechanical compression test. Journal of Food Engineering, 292, 110368.
Personnels impliqués
K. Ako, H. Bodiguel, C. de Loubens, F. Pignon.
E. Guilbert (post-doc), A. Vilotte (PhD), T. Ekoué (PhD), F. Doudiès (PhD INRAE STLO - LRP)
Collaborations
- Share
- Share on Facebook
- Share on X
- Share on LinkedIn