- Share
- Share on Facebook
- Share on X
- Share on LinkedIn
The Rheology and Processes Laboratory houses a microscopy platform equipped to study materials and flows, a fast confocal microscope, an optical tweezers for microrheology, and a rheomicroscope. We also benefit from the expertise of our various imaging partners on the UGA campus.

Microscopy for Materials and Flows
These microscopes are equipped with fluorescence, phase contrast, and crossed polarizers for the observation and characterization of materials.
A specialty of our microscopy activities involves the analysis of flows at the micrometer scale through the design of milli- and microfluidic chambers (access to the MicroFAB technical platform of LIPhy). These observations are conducted using fluorescence with high-sensitivity cameras (Hamamatsu ORCA Flash cameras) or high-speed cameras (Photron FASTCAM mini AX), and the data is processed with internally developed image analysis algorithms.
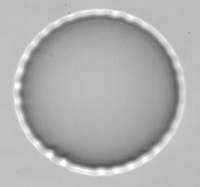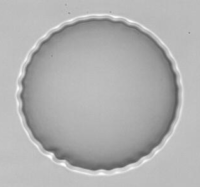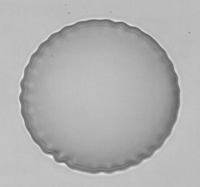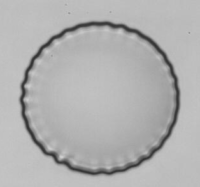
Observation of wrinkle instability on a microcapsule in an extensional flow.

Flow of a suspension within a porous medium.
Confocal Microscopy
The Rheology and Processes Laboratory also hosts a Nikon confocal microscope equipped with Yokogawa spinning disc technology, enabling rapid imaging of discrete planes in 3D samples or flows.
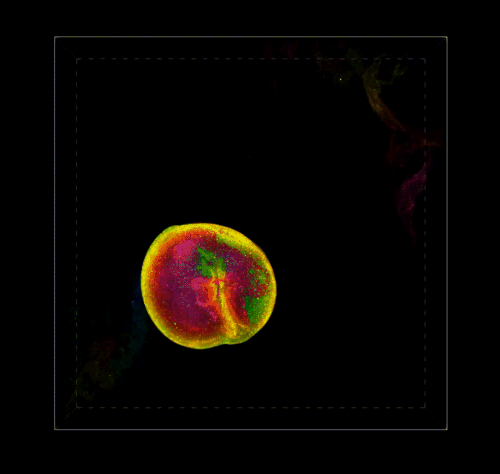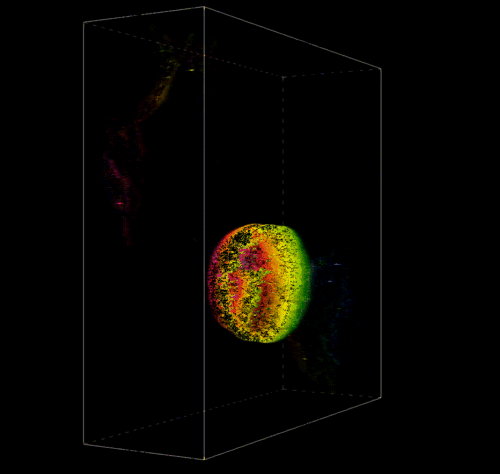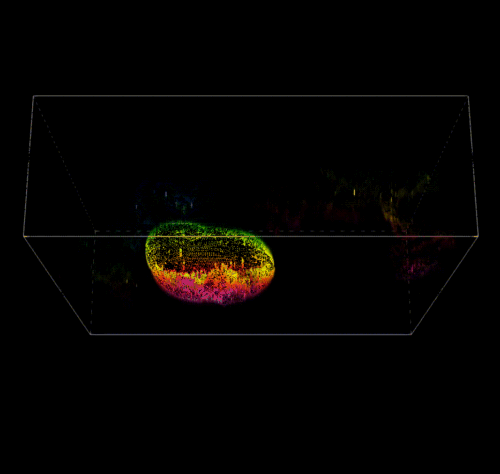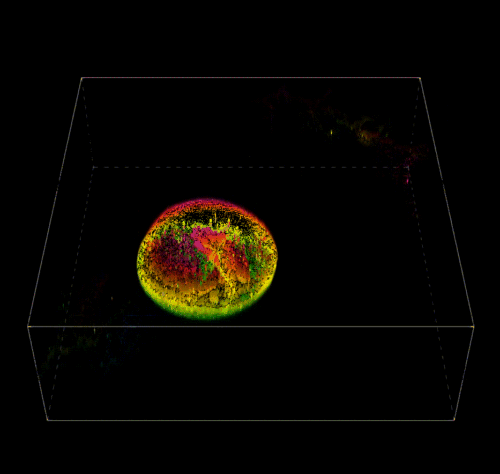
3D Reconstruction of a Pectin Shell.
Passive microrheology
Passive microrheology consists in quantifying the Brownian motion of micro-particles in a medium whose rheological properties are to be characterized.
![]()
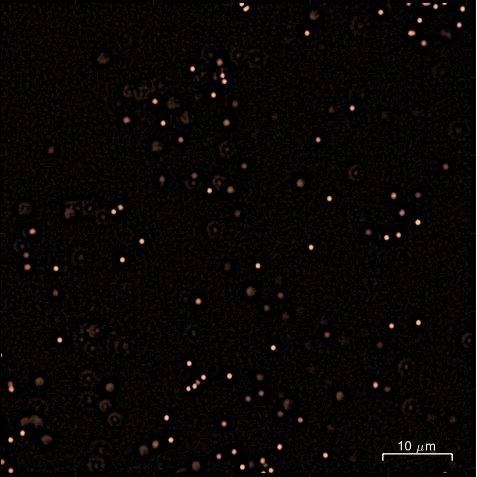
Confocal image of a liquid containing fluorescent tracer particles (diameter 0.5 µm) and magnified view of a single tracer particle and its Brownian trajectory.
The analysis of particle motion makes it possible to calculate their mean square displacement (MSD). In Newtonian liquids, viscosity can be measured over a wide range (from 10⁻³ Pa·s to 10 Pa·s). In purely elastic systems, the elastic modulus can be measured within a range of 0.1 to 20 Pa. The accessible frequency range extends from 0.1 to 20 Hz.
- The advantages of passive microrheology include:
- The very small sample volumes required,
- the ability to analyze heterogeneous samples,
- in-situ monitoring of rheological properties.
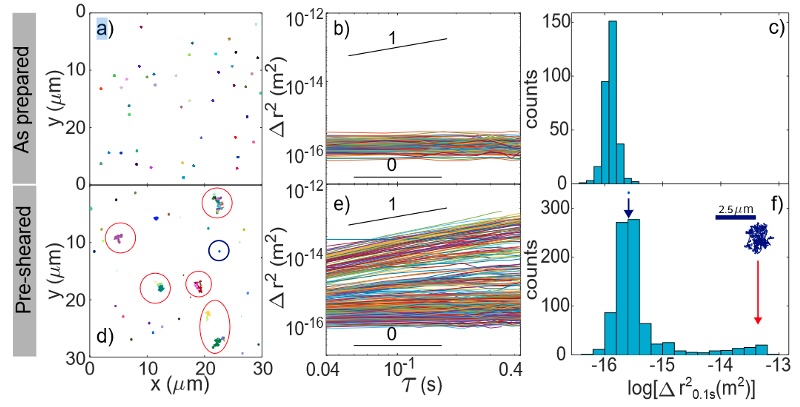
Example of microrheology results on gel samples [D. Milian et al., Biomacromolecules 2023]. As prepared the gel is purely elastic and the MSDs are uniform and time-independent. After a pre-shear, microcracks are visible and tracer particles located in these microcracks exhibit much higher MSD and a diffusive behavior.
Optical Tweezers and Microrheology
We are also equipped with our own optical tweezers setup, allowing us to trap microparticles and exert forces in the range of 1 to 100 pN on them, enabling the manipulation of objects (red blood cells, yeast) and conducting active microrheology experiments.
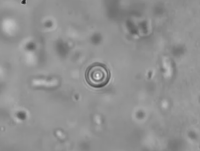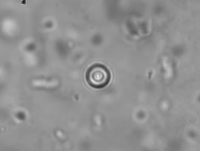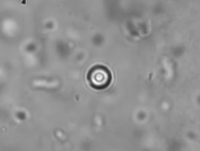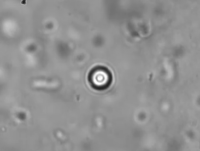
Active Microrheology in Pulmonary Mucus.
Rheo-Microscopy
One of our rheometers (MCR 501, Anton Paar) is equipped with an optical setup that allows for the coupling of rheometric measurements and sample observation.
Structuration sous cisaillement d'une suspension de colloïdes.
Imaging Partners
We also collaborate with the Materials Characterization Technology Platform and the electron microscopy facility at CERMAV for the morphological and structural study of soft matter: Scanning Electron Microscopy (SEM), Transmission Electron Microscopy (TEM), X-ray diffraction, Raman spectroscopy, and Tomography.
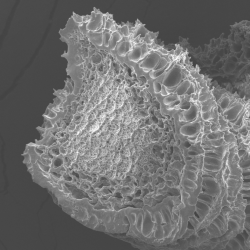
Observation by Scanning Electron Microscopy of a protein aggregate fiber obtained through co-injection.
- Share
- Share on Facebook
- Share on X
- Share on LinkedIn