- Share
- Share on Facebook
- Share on X
- Share on LinkedIn
Objectives
The composition of blood—a dense suspension of red blood cells accounting for 40 to 50% of its volume—makes it a complex fluid from a rheological perspective, leading to intricate flow structures within the circulatory system, particularly at the microcirculation scale.
Red blood cells exhibit complex dynamics even in simple flows due to their mechanical properties, not to mention the variability of these properties based on factors such as cell age or pathological conditions. Furthermore, interactions between red blood cells in dense suspensions, whether purely hydrodynamic or linked to aggregation properties, result in complex structures and rheological behaviors.
We address various questions related to the mechanisms of microcirculation through experimental studies at different scales, inspired by biological and medical challenges. These studies aim to advance the fundamental understanding of these mechanisms and may lead to applications in patient diagnosis and monitoring.
Projects
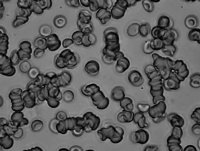
Structure and stability of red blood cell aggregates
(collaboration C. Wagner, Saarland University, PhD thesis M. Puthumana Melepattu)
At low shear rates, red blood cells aggregate due to bridging mechanisms and/or depletion forces related to the presence of proteins in plasma (notably fibrinogen), leading to the formation of structures called ‘rouleaux,’ which resemble stacks of coins. This reversible aggregation is responsible for the shear-thinning behavior of blood (blood viscosity is higher at lower flow velocities) and governs the sedimentation rate of blood—a classic test in hematology—due to the variability of fibrinogen levels in plasma (which increases in pathological conditions). The stability of these aggregates, determined by membrane interaction forces and the mechanical properties of red blood cells, influences the level of aggregation in capillaries and microcirculatory dynamics [1]. We study the mechanisms of red blood cell aggregation and disaggregation and have specifically developed a microfluidic measurement technique combined with AI analysis to characterize the aggregability of red blood cells under elongational flow [2], enabling the investigation of the influence of various parameters such as red blood cell aging. [3].
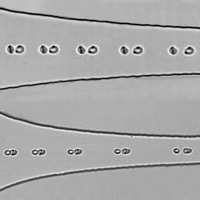
Kinetics of red cell aggregation and blood rheology
(collaboration C. Minetti, ULB Brussels, with support from CNES et ESA)
As part of the KRABS project (Kinetics of Red Cell Aggregation and Blood Structure-Rheology Link), supported by CNES and ESA, we are studying the kinetics of blood rheological changes associated with red blood cell aggregation, in connection with circulation abnormalities observed in astronauts during spaceflight. The project involves microgravity experiments conducted during parabolic flights aboard Novespace’s Airbus Zero-G and on the MASER 16 sounding rocket (flight scheduled for 2026) operated by the Swedish Space Corporation.

Traffic dynamics and instabilities of blood microcirculation
(collaborations G. Coupier, LIPhy Grenoble, N. Karst, Babson College, J. Geddes, Olin College, postdoc M. Alonzo, with LabEx Tec21 support)
Blood microcirculation, where exchanges between blood and tissues occur, involves complex networks of capillary vessels in which flow is highly heterogeneous in terms of flow velocity and hematocrit (red blood cell concentration) and can exhibit significant temporal fluctuations. These fluctuations result from complex rheological behaviors linked to the structuring of red blood cells in the vessels and phase separation phenomena occurring at network bifurcations. By combining experiments and modeling, we study the fundamental mechanisms (blood rheology, distribution at bifurcations) and the conditions under which multiple flow configurations emerge in complex networks, as well as their implications for microcirculation [4].
Rheological biomarkers of sickle cell disease
(collaboration C. Makowski, CHU Grenoble Alpes)
Sickle cell disease is an inherited red blood cell disorder characterized by stiffening, shape changes, and increased fragility of red blood cells due to an alteration in hemoglobin, leading to its polymerization under low oxygen partial pressure. The most severe symptom is the sickle cell vaso-occlusive crisis, which occurs when a large number of stiffened cells cause an obstruction in the microcirculation. There are few indicators to quantify the severity of the disease or to account for its phenotypic heterogeneity, and no reliable predictors of vaso-occlusive events. We are conducting a clinical study in partnership with CHU Grenoble Alpes to assess the relevance of the distribution of red blood cell rheological properties within a sample for patient diagnosis and monitoring. This is achieved through direct deformability measurements using microfluidics and testing a technique based on fluorescent probes, which has undergone initial proof-of-concept validation [5].
References
[1] J. Martin-Wortham, Exploration de la dynamique de l’Agrégation des Globules Rouges dans la Microcirculation, thèse de doctorat de l’Université Grenoble Alpes (2023)
[2] M. Puthumana Melepattu, G. Maîtrejean, T. Podgorski, Dissociation of red blood cell aggregates in extensional flow, Physical Review Fluids 9, L071101 (2024)
[3] M. Puthumana Melepattu, G. Maîtrejean, C. Wagner, T. Podgorski, Influence of cell density and in-vivo aging on erythrocyte aggregability: Dissociation dynamics in extensional flow, submitted to Journal of Biomechanics (2024)
[4] M. Alonzo, N. J. Karst, T. Podgorski, J. B Geddes, G. Coupier, Spatio-Temporal Instabilities of Blood Flow in a Model Capillary Network, Physical Review Fluids 9, 104401 (2024)
[5] A. Briole, T. Podgorski, B. Abou, Molecular rotors as intracellular probes of red blood cell stiffness, Soft Matter 17, 4525-4537 (2021)
Team members involved
Thomas Podgorski (DR CNRS)
Guillaume Maîtrejean (MCF UGA)
Julie Martin-Wortham (PhD thesis, 2023)
Midhun Puthumana Melepattu (PhD thesis, 2025)
Internship and PhD opportunities
We welcome students from a variety of backgrounds (Bachelor’s, Master’s, engineering schools, or technical programs) with diverse and motivated profiles, eager to work on projects at the interface of fluid mechanics, biophysics, and biomedical applications. Do not hesitate to send a spontaneous application, even outside the specific internship opportunities listed below.
Current opportunities :
- Kinetics of Red Blood Cell aggregation and Blood rheology
- Impact of Red Blood Cell Deformability on Aggregation
- Share
- Share on Facebook
- Share on X
- Share on LinkedIn