- Share
- Share on Facebook
- Share on X
- Share on LinkedIn
We are developing and characterising nanomaterials obtained using an electrospinning process for various tissue engineering applications.
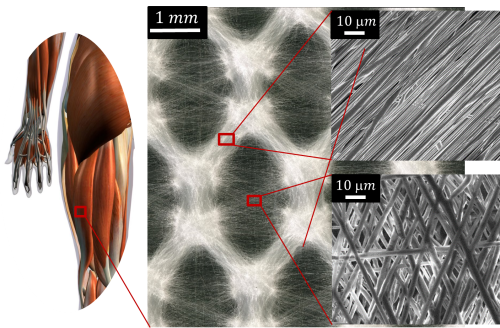
Nanofibrous honeycomb structure of biocompatible, bioresorbable polymer.
Objectives
The aim of this research area is to develop biocompatible and potentially bioresorbable biopolymer-based materials with controlled mechanical properties. These structures can be functionalized on the surface to give them new properties for use. The applications targeted are the development of cell growth implants, injectable suspensions for the treatment of osteoarthritis of the knee, and antibacterial materials.
Results
-
Biomimetic hyperelastic implants for soft tissue reconstruction (collaboration with IBMM, TIMC)
These implants must be both biodegradable and biomimetic, enabling them to be colonised by the host tissue while avoiding the need for surgery to remove the implant. Fibrous structures have been developed that combine mechanical properties (hyperelasticity and anisotropy) as close as possible to those of the targeted tissue, while allowing cells to move around. New polymers have been synthesised, with degradation rates and mechanical properties that can be modulated by their different structures (linear or star chain) and which can carry UV-sensitive groups. They were shaped by electrospinning to create networks of nanofibres with controlled architecture.
These structured membranes were seeded with human fibroblast cells in order to characterise the affinity of the structures with the cells and their regenerative potential. Finally, the mechanical properties of these implants were characterised, both experimentally and by numerical simulation, as were the mechanisms of cell colonisation.
-
Injectable suspensions of chitosan nanofibre/chondrocyte composites for the treatment of osteoarthritis of the knee (collaboration with Grenoble Alpes University Hospital)
Osteoarthritis is characterised by the destruction of cartilage, leading to inflammation of the membrane that lines the inside of the joint. Our strategy is to develop fibrous chitosan structures that are biocompatible with native tissue, seeded with chondrocytes, the cells that make up cartilage, and made into injectable suspensions.
Cell viability, adhesion and proliferation were studied and compared as a function of substrate morphology and chitosan neutralisation. Our studies showed that cells grew better on fibres than on films. A chondrocyte survival rate of over 80% was observed on all types of chitosan support. However, cell viability and proliferation were enhanced when cells adhered to small-diameter fibres.
-
Development, functionalisation by enzyme immobilisation and characterisation of 3D fibrous nanomaterials for antibacterial applications in tissue engineering (collaboration with CERMAV)
Fibres based on mixtures of PCL/keratin/Alpha-lactalbumin are produced and then functionalised by immobilisation of lysozymes, enzymes with the ability to destroy the bacterial wall. In order to prevent any denaturation of the enzyme during the electrospinning process, functionalization is carried out once the fibres have been formed, either by chemical surface modification enabling the introduction of a reactive functional group complementary to a protein surface function, or by having introduced this functional group during fibre formation.
References
- H. Mondésert, F. Bossard, D. Favier, "Anisotropic electrospun honeycomb polycaprolactone scaffolds: Elaboration, morphological and mechanical properties", journal of the mechanical behavior of biomedical materials 113, 2021, 104124; https://doi.org/10.1016/j.jmbbm.2020.104124
- L. Gangolphe, C.Y. Leon-Valdivieso, B. Nottelet, S Déjean, A. Bethry, C. Pinese, F. Bossard, X. Garric, " Electrospun microstructured PLA-based scaffolds featuring relevant anisotropic, mechanical and degradation characteristics for soft tissue engineering", Materials Science & Engineering C 129, 2021, 112339; https://doi.org/10.1016/j.msec.2021.112339
- C.E. Garcia Garcia, B. Lardy, F. Bossard, F.A. Soltero Martínez, M. Rinaudo, "Chitosan based biomaterials for cartilage tissue engineering: Chondrocyte adhesion and proliferation", Food Hydrocolloids for Health, 2021, 100018; https://doi.org/10.1016/j.fhfh.2021.100018
Staff involved :
- Share
- Share on Facebook
- Share on X
- Share on LinkedIn