- Share
- Share on Facebook
- Share on X
- Share on LinkedIn
Spontaneous contraction of polysaccharide gels
The spontaneous contraction of gels observed under certain storage conditions is related to the thermodynamic properties of the units and aggregates structuring the single-component gel. This is the result highlighted in this study, using a polysaccharide, kappa-carrageenan (kC), as the sole gel-forming component. We found that kC gels expel solvent depending on its salt composition—a phenomenon known as syneresis—when the gel is stored within a specific temperature range.
To study the thermal stability of kC gels, we developed a method to investigate the thermodynamic properties of the polysaccharide in solution using rheology. We measured the viscosity of kC solutions as a function of polysaccharide concentration at different temperatures. For each temperature, we obtained a critical concentration (C*) which is a function of the hydrodynamic radius (Rh) of the polysaccharide. This provides the relationship between solution temperature and a characteristic parameter of polysaccharide size. From this (T, Rh) relationship, we deduce the thermodynamic cause of polysaccharide gel contraction. The contraction effects are kinetic in nature.
The results of this study would not have been possible without the following principle: “For any simple or compound pure liquid subjected to a temperature variation, there exists a temperature at which the viscosity of the liquid reaches a critical minimum value.” The basis of this concept is detailed in a publication where its application to water showed a minimum viscosity at 100 °C ± 2 °C under atmospheric pressure. We can now predict the thermal stability of gels from any polymer by measuring (T, Rh).
Ionic properties of carrageenans
For decades, polysaccharide experts considered the ionic properties of carrageenans to depend almost exclusively on the number of negatively charged functional groups carried by the monomer. It is well-known among specialists that the ionic affinity of kC for monovalent potassium is linked to the monovalence of the kC monomer, the affinity of iota-carrageenan (iC) for divalent calcium is linked to the divalence of the iC monomer, and the affinity of lambda-carrageenan (lC) for trivalent iron is linked to the trivalence of the lC monomer.
We showed that the ionic activity of carrageenans depends not only on the number of charged functional groups but also on their position on the monomer unit. From this, we highlighted the role of the anhydro bridge in ion selectivity. When the charged functional group is on the monomer unit with an anhydro bridge, the reactivity with potassium is lower than when the monomer unit lacks an anhydro bridge. We also demonstrated the difference in solubility between cellulose, which has no anhydro bridge, and polysaccharides containing one or more anhydro bridges along the chain. The anhydro bridge is a functional group favorable for polysaccharide solubilization.
Pellet–helix transition of carrageenan gels
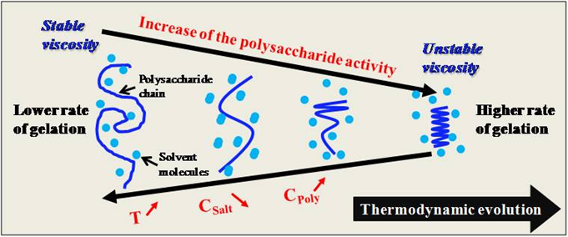
This study demonstrates the role of helix formation during the coil–helix transition in gel stability. Water trapped within the coil is expelled to allow the coil–helix transition. This transition is clearly measurable by rheology, as it directly affects the viscosity and viscoelastic moduli of the system, which changes from a liquid to a gel state. We showed how structuring occurs layer by layer and throughout the bulk during the gelation and aging process, while highlighting the effects of temperature, ionic strength, and polysaccharide concentration on the coil–helix transition.
References
Ako, K., Elmarhoum, S., & Munialo, C. D. (2022). The determination of the lower critical concentration temperature and intrinsic viscosity: The syneresis reaction of polymeric gels. Food Hydrocolloids, 124, 107346.
Elmarhoum, S., Mathieu, S., Ako, K., & Helbert, W. (2023). Sulfate groups position determines the ionic selectivity and syneresis properties of carrageenan systems. Carbohydrate Polymers, 299, 120166.
Elmarhoum, S., Ako, K., Munialo, C. D., & Rharbi, Y. (2023). Helicity degree of carrageenan conformation determines the polysaccharide and water interactions. Carbohydrate Polymers, 314, 120952.
Personnels impliqués
Y. Rharbi, K. Ako
S. Elmarhoum (PhD)
Remerciements
Nous remercions Markem-Imaje pour leur travail de recherche et le développement du dispositif expérimental utilisé dans ce travail, ainsi que pour leur expertise et leurs contributions inestimables qui ont été fondamentales pour ces résultats.
- Share
- Share on Facebook
- Share on X
- Share on LinkedIn